Conductivity experiments for electrolyte formulations and their automated analysis
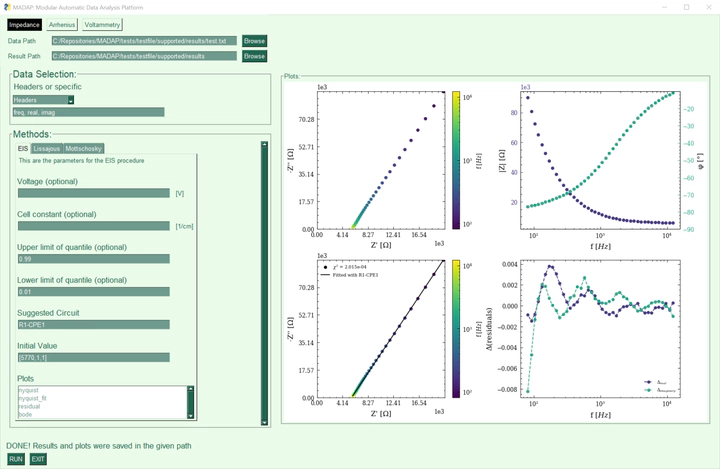 Image credit: Fuzhan Rahmanian
Image credit: Fuzhan RahmanianAbstract
Electrolytes are considered crucial for the performance of batteries, and therefore indispensable for future energy storage research. This paper presents data that describes the effect of the electrolyte composition on the ionic conductivity. In particular, the data focuses on electrolytes composed of ethylene carbonate (EC), propylene carbonate (PC), ethyl methyl carbonate (EMC), and lithium hexafluorophosphate (LiPF6). The mass ratio of EC to PC was varied, while keeping the mass ratio of (EC + PC) and EMC at fixed values of 3:7 and 1:1. The conducting salt concentration was also varied during the study. Conductivity data was obtained from electrochemical impedance spectroscopy (EIS) measurements at various temperatures. Based on the thus obtained temperature series, the activation energy for ionic conduction was determined during the analysis. The data is presented here in a machine-readable format and includes a Python package for analyzing temperature series of electrolyte conductivity according to the Arrhenius equation and EIS data. The data may be useful e.g. for the training of machine learning models or for reference prior to experiments.